Epidemiology and diagnostic criteria
Neurofibromatosis type 1 (NF1) is a genetic disease of autosomal dominant inheritance, linked to a mutation in the NF1 gene encoding neurofibromin.
The genetics of neurofibromatosis is included in a dedicated chapter. Its prevalence is 1/40001–4 and its incidence estimated at one in 3000 births1,2,5–7.
Its phenotypic expression is variable even between members of a family. Its penetrance is complete at the age of 8 years: 97% of patients with NF1 meet the diagnostic criteria at 8 years8.
The diagnosis is based on the combination of at least 2 of the 7 NIH criteria from 19889 revised in 202110, and summarized below. They differ depending on whether the patient has one of his two parents also affected.
REVISED DIAGNOSTIC CRITERIA FOR NF1 – 2021
A. The diagnostic criteria for NF1 are met in an individual who does not have a parent diagnosed with NF1 if two or more of the following are present:
- Six or more "café-au-lait" macules over 5 mm in greatest diameter in prepubertal individuals and over 15 mm in greatest diameter in post-pubertal individuals.
- Freckling in the axillary or inguinal region* and **.
- Two or more neurofibromas of any type or one plexiform neurofibroma.
- Optic pathway glioma.
- Two or more iris Lisch nodules identified by slit lamp examination or two or more choroidal abnormalities (CAs)—defined as bright, patchy nodules imaged by optical coherence tomography (OCT)/near-infrared reflectance (NIR) imaging.
- A distinctive osseous lesion such as sphenoid dysplasia***, anterolateral bowing of the tibia, or pseudarthrosis of a long bone.
- A heterozygous pathogenic NF1 variant with a variant allele fraction of 50% in apparently normal tissue such as white blood cells.
B. A child of a parent who meets the diagnostic criteria specified in A merits a diagnosis of NF1 if one or more of the criteria in A are present.
**At least one of the two pigmentary findings (café-au-lait macules or freckling) should be bilateral.
***Sphenoid wing dysplasia is not a separate criterion in case of an ipsilateral orbital plexiform neurofibroma.
-
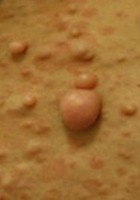
- Cutaneous neurofibromas (adult)
-
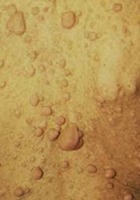
- Cutaneous neurofibromas (adult)
-
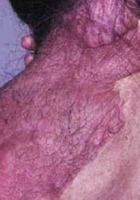
- Posterior cervical plexiform neurofibroma (adult)
-
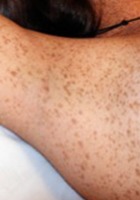
- Freckling in the axillary region (adult)
-
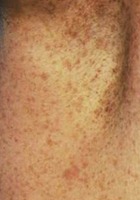
- Freckling in the axillary region (adult)
-
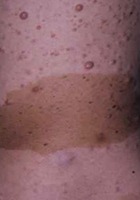
- "Café-au-lait" macules associated with cutaneous neurofibromas
-
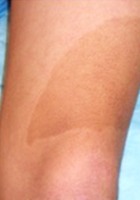
- Large "café-au-lait" macules (adult)
-
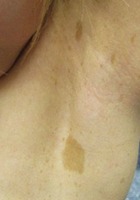
- "Café-au-lait" macules associated with freckling in the bilateral axillary region(child)
-
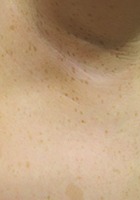
- "Café-au-lait" macules associated with freckling in the bilateral axillary region(child)
-
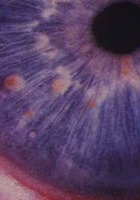
- Lisch nodules (slit lamp examination)
Complications
The disease complications are numerous, benign or malignant, somatic and psychological, and appear throughout the lifespan11. However, one of the peculiarities of NF1 remains its variability of expression from one patient to another and even within a family (where the genetic abnormality is identical). This makes it impossible to predict the clinical course of an affected child.
There are different types of neurofibromas: dermal (>95% of patients)12,13, peripheral nodular (at least 20% of patients)12and nodular orplexiform (20% of patients)14. The main risk, inherent in nodular and plexiform neurofibromas, is the transformation into a malignant peripheral nerve sheathtumour (MPNST, formerly known as malignant nerve sheathtumour). The cumulative risk of developing MPNST during a patient's lifetime has been estimated to be 8-16%. It occurs mostly between 20 and 35 years of age15–19.
This risk is increased in patients with an NF1 phenotype, defined by the presence of subcutaneous neurofibromas: in case of subcutaneous neurofibromas, the risk of developing an internal plexiform neurofibroma or MPNST is multiplied by 3, and in case of plexiform neurofibroma, the risk of developing an MPNST is multiplied by 2020. The tumour may be aggressive17 , with a decreased life expectancy16.
Metabolic imaging such as PET-MRI or PET-scanner can diagnose a neurofibroma in the dysplastic stage and thus improve the prognosis through early surgery. Neurofibromas can also have a significant aesthetic and functional impact.
Optic pathway glioma21–25 is a benign tumour with low malignancy potential present in 15-20% of children with NF121, 26–30. On the other hand, its local extension can lead to exophthalmos, decreased visual acuity, or compression of the optic chiasma with possible repercussions on the gonadotropic hypothalamic-pituitary axis and early puberty30, 31.
Orthopaedic disorders are classic: congenital dysplasia of long bones (7.2% of patients)32, sphenoidal dysplasia (1 to 7% of patients)33 and scoliosis (10 to 28% of patients)34.
Congenital dysplasia of long bones leads to deformities and an over-risk of fracture, with pseudarthrosis constitution in 2 to 3.6% of patients32, 35.
Sphenoidal dysplasia can be secondary to plexiform neurofibroma36, and can lead to pulsatile exophthalmos without decreased visual acuity and a hernia of the temporal lobe in the eyeball in case of total absence of sphenoid bone37.
Scoliosis, the most common orthopaedic manifestation, is often associated with vertebral dysplasia38.
Patients with NF1 appear to be at increased risk of weakening osteopathy, with osteopenia presentin 48% of patients or osteoporosis in 25%39, 40.
Early stunting is present in one-third of children41, precocious puberty can also occur33, 42. Precocious puberty is defined by the appearance of sexual criteria before the age of 7-8 years in girls and 9-10 years in boys.
Epilepsy and its developmental repercussions are the most severe central neurological complication. Epilepsy affects 8% of patients56, 57, and is resistant to treatment in 30% of cases58. In the latter cases, it can be associated with severe mental retardation.
Moderate cognitive impairment, outside of epilepsy, is the most common neurological complication59, with an intelligence quotient in the lower middle part, behavioural disorders, and specific learning difficulties (visuospatial and visuomotor disorders, language disorders, fine and gross motor disorders, and executive function disorders)5, 59–64. Other pathologies have also been associated with NF1, including attention deficit/hyperactivity, autistic, and psychosocial disorders62, 64.
Finally, patients with NF1 have an increased risk of neoplasia65.
Regarding the risk of breast cancer, particularly assessed in the population of patients with NF1, it occurs in women under 50 years of age66–70 with a higher mortality rate71. These characteristics justify the need of systematic annual screening by combining mammography-ultrasounds72 and breast MRIs from the age of 30 years in women with NF1.
MPNSTs account for 60% of cancers in this population, but patients can also develop other tumours on the neurological spectrum: brain tumours (including gliomas of the optic tract), gliomas of the brainstem73, or gastrointestinal neuroendocrine tumours, which often occur at a younger age than that of the general population and that are often multiple and regularly located in the small intestine74, 75.
The chronic psychological repercussions of NF1 have been the subject of two studies evaluating anxio-depressive disorders in adults76, and children77 : 19% of adults reported depressive symptoms and 15% anxiety disorders76. In adults, the associations between the severity of anxio-depressive symptoms and the severity of the disease (calculated by a modified version of the Riccardi score78) or the visibility of skin lesions (calculated according to the Ablon score79) are significant (p<0.001)76.
Sleep disturbances in patients with NF1 have also been evaluated in one study in adults80 and another one in children64. The mean scores of ESS (Epworth Sleepiness Scale)81 and PSQI (Pittsburgh Sleep Quality Index)82 which assess sleep disturbances, are abnormally high in these80, with no correlation between the two scores.
In children with NF1, conduct problems (p≤0.05), hyperactivity (p≤0.01), emotional fragility (p≤0.01), and total difficulty score (p ≤0.01) are significantly increased and correlated with sleep disturbances64. These psychological and sleep impacts, associated with chronic pain, significantly contribute to the impairment of the quality of life of patients with NF1 83–85. An adaptation of quality-of-life scores (Skindex-16) and burden are underway for NF1: cNF-SKindex86 and Burden of NF1, respectively.
The long-term management of these patients must integrate this dimension.
The risk of mortality is increased in patients with NF1. Compared to the general population, their life expectancy is shortened by 10 to 15 years15, 66, 67, 71, 87–89. In France, the last NF1 mortality study dates from 201189, and concerned data from 1985 patients between 1980 and 2005.
All-cause mortality was significantly higher in the NF1 patient cohort, with a SMR (standardized mortality ratio) of 2.02 (1.6-2.6) (p<0.0001). The main cause of death, available for 58 patients (86.6% of patients who died), was MPNST.
Main differential diagnosis
The main differential diagnosis of neurofibromatosis type 1 (NF1) is Legius syndrome. Like the diagnostic criteria for NF1, those for Legius syndrome are divided into two scenarios (A and B) depending on the absence (A) or presence (B) of an affected parent.
Thus, the diagnosis of Legius syndrome is made under the following conditions:
A. The diagnostic criteria for Legius syndrome are met in an individual who does not have a parent diagnosed with Legius syndrome if the following criteria are present:
- Six or more "café-au-lait" macules bilaterally distributed and no other NF1-related diagnostic criteria except for axillaryor inguinal freckling*.
- A heterozygous pathogenic variant in SPRED1 with a variant allele fraction of 50% in apparently normal tissue such as white blood cells.
Patient care
For the most symptomatic forms, NF1 requires multidisciplinary and multi-professional monitoring over the longterm, with specific research of the various above-mentioned complications and appropriate support, sometimes from an early age (with learning support, for instance).
This care is routinely organized near patients with reference to the appropriate referralcentre of neurofibromatosis, when necessary.
The National Diagnostic and Care Protocols ("Protocoles Nationaux de Diagnostic et de Soin", PNDS) was updated in 2021, and it is available on the French High Authority of Health website or on the page of useful documents for healthcare professionals.
While there is no treatment for the genetic mutation responsible for NF1, there are multiple treatments for the different manifestations and complications of the disease.
These complications are most often not specific to NF1, and their management does not differ from the same situations encountered in patients without NF1. However, several cases need to be clarified.
Pain is a very common symptom of NF1 and can accompany, or even reveal, many complications. The neuropathic component of pain is preponderant when it is secondary to compressive and/or transformed internal neurofibromas. The use of active treatments on neuropathic pain, in addition to conventional analgesics, is therefore essential for patients with NF1. These treatments include drugs from the family of antiepileptics and/or antidepressants.
Pain management can be improved by learning non-medicinal techniques, particularly when pain becomes chronic and resistant to "conventional" treatments. The help of teams specialising in the study and treatment of pain is sometimes required. Finally, we must not overlook the negative emotional impact of this pain, which can lead to the provision of appropriate psychological support.
For adult patients, many hospitals offer specialised pain management. Referral and competence centres most often work with a pain management team from the same hospital. For patients suffering from chronic pain in the Paris region, they can contact a specialised structure within the AP-HP.
The assessment and management of children's pain is complex and most often involves multidisciplinary and multi-professional teams. Various websites can help parents, but healthcare professionals may also contribute to the assessment and management of pain, such as Pediadol or Dolomio, which are a group of experts in child and adolescent pain.
The use of a specialised team may sometimes be necessary. Thus, referral and competence centres for the management of children with NF1 often work closely with teams specialised in the treatment of pain.
Malignant peripheral nerve sheaths tumours (MPNSTs) are serious complications whose prognosis depends on the possibility of performing a broad surgical excision. After surgical treatment, when possible, adjuvant radiotherapy is most often offered. In case of a locally advanced and inoperable disease, or in the metastatic stage, poly-chemotherapy is proposed.
Cutaneous neurofibromas rarely evolve into MPNST, but they can be responsible for major aesthetic discomfort. The only treatments currently available are interventional treatments: CO2 laser, conventional surgical excision, and electro-dissection. Aesthetic results depend on the quality of healing and the number of lesions to be treated.
MEK inhibitors have recently emerged as part of NF1, including trametinib and selumetinib. Several studies have shown their efficacyfor the treatment for symptomatic and inoperable internal neurofibromas90–93. Recently, selumetinib has obtained a Marketing Authorization Application (MAA) for the following indication: inoperable plexiform neurofibromas in children.
This MAA replaces the Temporary Authorization for Use (Autorisation Temporaire d'Utilisation, ATU) initially required. These treatments can be prescribed in specialised centres after collegial discussion. Real-life usage data and the development of new molecules and formulations could expand their fields of use in the future.
Bibliographic references
- Lammert M, Friedman JM, Kluwe L, Mautner VF. Prevalence of neurofibromatosis 1 in German children at elementary school enrollment. Arch Dermatol. 2005;141(1):71-74. doi:10.1001/archderm.141.1.71.
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A. 2010;152A(2):327-332. doi:10.1002/ajmg.a.33139.
- Huson SM, Compston DA, Clark P, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. I. Prevalence, fitness, mutation rate, and effect of parental transmission on severity. J Med Genet. 1989;26(11):704-711. doi:10.1136/jmg.26.11.704.
- Kallionpää RA, Uusitalo E, Leppävirta J, Pöyhönen M, Peltonen S, Peltonen J. Prevalence of neurofibromatosis type 1 in the Finnish population. Genet Med Off J Am Coll Med Genet. 2018;20(9):1082-1086. doi:10.1038/gim.2017.215.
- Huson SM, Compston DA, Harper PS. A genetic study of von Recklinghausen neurofibromatosis in south east Wales. II. Guidelines for genetic counselling. J Med Genet. 1989;26(11):712-721. doi:10.1136/jmg.26.11.712.
- Poyhonen M, Kytölä S, Leisti J. Epidemiology of neurofibromatosis type 1 (NF1) in northern Finland. J Med Genet. 2000;37(8):632-636. doi:10.1136/jmg.37.8.632.
- Uusitalo E, Leppävirta J, Koffert A, et al. Incidence and mortality of neurofibromatosis: a total population study in Finland. J Invest Dermatol. 2015;135(3):904-906. doi:10.1038/jid.2014.465.
- DeBella K, Szudek J, Friedman JM. Use of the national institutes of health criteria for diagnosis of neurofibromatosis 1 in children. Pediatrics. 2000;105(3 Pt 1):608-614. doi:10.1542/peds.105.3.608.
- Neurofibromatosis. Conference statement. National Institutes of Health Consensus Development Conference. Arch Neurol. 1988;45(5):575-578.
- Legius E, Messiaen L, Wolkenstein P, Pancza P, Avery RA, Do G. Revised diagnostic criteria for neurofibromatosis type 1 and Legius syndrome: an international consensus recommendation. Genet Med. Published online May 19, 2021. doi:10.1038/s41436-021-01170-5
- Bergqvist C, Servy A, Valeyrie-Allanore L, et al. Neurofibromatosis 1 French national guidelines based on an extensive literature review since 1966. Orphanet J Rare Dis. 2020;15(1):37. doi:10.1186/s13023-020-1310-3.
- Duong TA, Bastuji-Garin S, Valeyrie-Allanore L, Sbidian E, Ferkal S, Wolkenstein P. Evolving pattern with age of cutaneous signs in neurofibromatosis type 1: a cross-sectional study of 728 patients. Dermatol Basel Switz. 2011;222(3):269-273. doi:10.1159/000327379.
- Ortonne N, Wolkenstein P, Blakeley JO, et al. Cutaneous neurofibromas: Current clinical and pathologic issues. Neurology. 2018;91(2 Suppl 1):S5-S13. doi:10.1212/WNL.0000000000005792.
- Darrigo LG, Geller M, Bonalumi Filho A, Azulay DR. Prevalence of plexiform neurofibroma in children and adolescents with type I neurofibromatosis. J Pediatr (Rio J). 2007;83(6):571-573. doi:10.2223/JPED.1718.
- Uusitalo E, Rantanen M, Kallionpää RA, et al. Distinctive Cancer Associations in Patients With Neurofibromatosis Type 1. J Clin Oncol Off J Am Soc Clin Oncol. 2016;34(17):1978-1986. doi:10.1200/JCO.2015.65.3576.
- Evans DGR, Baser ME, McGaughran J, Sharif S, Howard E, Moran A. Malignant peripheral nerve sheath tumours in neurofibromatosis 1. J Med Genet. 2002;39(5):311-314. doi:10.1136/jmg.39.5.311.
- Ferner RE, Gutmann DH. International consensus statement on malignant peripheral nerve sheath tumors in neurofibromatosis. Cancer Res. 2002;62(5):1573-1577.
- Ducatman BS, Scheithauer BW, Piepgras DG, Reiman HM, Ilstrup DM. Malignant peripheral nerve sheath tumors. A clinicopathologic study of 120 cases. Cancer. 1986;57(10):2006-2021. doi:10.1002/1097-0142(19860515)57:10<2006::aid-cncr2820571022>3.0.co;2-6.
- Evans DGR, Huson SM, Birch JM. Malignant peripheral nerve sheath tumours in inherited disease. Clin Sarcoma Res. 2012;2(1):17. doi:10.1186/2045-3329-2-17.
- Tucker T, Wolkenstein P, Revuz J, Zeller J, Friedman JM. Association between benign and malignant peripheral nerve sheath tumors in NF1. Neurology. 2005;65(2):205-211. doi:10.1212/01.wnl.0000168830.79997.13.
- Listernick R, Charrow J, Greenwald M, Mets M. Natural history of optic pathway tumors in children with neurofibromatosis type 1: a longitudinal study. J Pediatr. 1994;125(1):63-66. doi:10.1016/s0022-3476(94)70122-9.
- Slamovits TL. Von Recklinghausen neurofibromatosis: II. Incidence of optic gliomata. Ophthalmology. 1985;92(5):714-715.
- Kornreich L, Blaser S, Schwarz M, et al. Optic pathway glioma: correlation of imaging findings with the presence of neurofibromatosis. AJNR Am J Neuroradiol. 2001;22(10):1963-1969.
- Deliganis AV, Geyer JR, Berger MS. Prognostic significance of type 1 neurofibromatosis (von Recklinghausen Disease) in childhood optic glioma. Neurosurgery. 1996;38(6):1114-1118; discussion 1118-1119. doi:10.1097/00006123-199606000-00010.
- Listernick R, Darling C, Greenwald M, Strauss L, Charrow J. Optic pathway tumors in children: the effect of neurofibromatosis type 1 on clinical manifestations and natural history. J Pediatr. 1995;127(5):718-722. doi:10.1016/s0022-3476(95)70159-1.
- Listernick R, Charrow J, Greenwald MJ, Esterly NB. Optic gliomas in children with neurofibromatosis type 1. J Pediatr. 1989;114(5):788-792. doi:10.1016/s0022-3476(89)80137-4.
- Lewis RA, Gerson LP, Axelson KA, Riccardi VM, Whitford RP. von Recklinghausen neurofibromatosis. II. Incidence of optic gliomata. Ophthalmology. 1984;91(8):929-935. doi:10.1016/s0161-6420(84)34217-8.
- Lund AM, Skovby F. Optic gliomas in children with neurofibromatosis type 1. Eur J Pediatr. 1991;150(12):835-838. doi:10.1007/BF01955002.
- Prada CE, Hufnagel RB, Hummel TR, et al. The Use of Magnetic Resonance Imaging Screening for Optic Pathway Gliomas in Children with Neurofibromatosis Type 1. J Pediatr. 2015;167(4):851-856.e1. doi:10.1016/j.jpeds.2015.07.001.
- Blazo MA, Lewis RA, Chintagumpala MM, Frazier M, McCluggage C, Plon SE. Outcomes of systematic screening for optic pathway tumors in children with Neurofibromatosis Type 1. Am J Med Genet A. 2004;127A(3):224-229. doi:10.1002/ajmg.a.20650.
- Laue L, Comite F, Hench K, Loriaux DL, Cutler GB, Pescovitz OH. Precocious puberty associated with neurofibromatosis and optic gliomas. Treatment with luteinizing hormone releasing hormone analogue. Am J Dis Child 1960. 1985;139(11):1097-1100. doi:10.1001/archpedi.1985.02140130035025.
- Boulanger JM, Larbrisseau A. Neurofibromatosis type 1 in a pediatric population: Ste-Justine’s experience. Can J Neurol Sci J Can Sci Neurol. 2005;32(2):225-231. doi:10.1017/s0317167100004017.
- Pinson S, Créange A, Barbarot S, et al. [Recommendations for the treatment of neurofibromatosis type 1]. J Fr Ophtalmol. 2002;25(4):423-433.
- Akbarnia BA, Gabriel KR, Beckman E, Chalk D. Prevalence of scoliosis in neurofibromatosis. Spine. 1992;17(8 Suppl):S244-248. doi:10.1097/00007632-199208001-00005.
- Ferner RE. Neurofibromatosis 1. Eur J Hum Genet EJHG. 2007;15(2):131-138. doi:10.1038/sj.ejhg.5201676.
- Arrington DK, Danehy AR, Peleggi A, Proctor MR, Irons MB, Ullrich NJ. Calvarial defects and skeletal dysplasia in patients with neurofibromatosis Type 1. J Neurosurg Pediatr. 2013;11(4):410-416. doi:10.3171/2013.1.PEDS12409.
- Ferner RE. Neurofibromatosis 1 and neurofibromatosis 2: a twenty first century perspective. Lancet Neurol. 2007;6(4):340-351. doi:10.1016/S1474-4422(07)70075-3.
- Ramachandran M, Tsirikos AI, Lee J, Saifuddin A. Whole-spine magnetic resonance imaging in patients with neurofibromatosis type 1 and spinal deformity. J Spinal Disord Tech. 2004;17(6):483-491. doi:10.1097/01.bsd.0000133466.97241.50.
- Brunetti-Pierri N, Doty SB, Hicks J, et al. Generalized metabolic bone disease in Neurofibromatosis type I. Mol Genet Metab. 2008;94(1):105-111. doi:10.1016/j.ymgme.2007.12.004.
- Lammert M, Kappler M, Mautner V-F, et al. Decreased bone mineral density in patients with neurofibromatosis 1. Osteoporos Int J Establ Result Coop Eur Found Osteoporos Natl Osteoporos Found USA. 2005;16(9):1161-1166. doi:10.1007/s00198-005-1940-2.
- Vassilopoulou-Sellin R, Woods D, Quintos MT, Needle M, Klein MJ. Short stature in children and adults with neurofibromatosis. Pediatr Nurs. 1995;21(2):149-153.
- Virdis R, Sigorini M, Laiolo A, et al. Neurofibromatosis type 1 and precocious puberty. J Pediatr Endocrinol Metab JPEM. 2000;13 Suppl 1:841-844. doi:10.1515/jpem.2000.13.s1.841.
- Lama G, Graziano L, Calabrese E, et al. Blood pressure and cardiovascular involvement in children with neurofibromatosis type1. Pediatr Nephrol Berl Ger. 2004;19(4):413-418. doi:10.1007/s00467-003-1397-5.
- Fossali E, Signorini E, Intermite RC, et al. Renovascular disease and hypertension in children with neurofibromatosis. Pediatr Nephrol Berl Ger. 2000;14(8-9):806-810. doi:10.1007/s004679900260.
- Virdis R, Balestrazzi P, Zampolli M, Donadio A, Street M, Lorenzetti E. Hypertension in children with neurofibromatosis. J Hum Hypertens. 1994;8(5):395-397.
- Tedesco MA, Di Salvo G, Ratti G, et al. Arterial distensibility and ambulatory blood pressure monitoring in young patients with neurofibromatosis type 1. Am J Hypertens. 2001;14(6 Pt 1):559-566. doi:10.1016/s0895-7061(00)01303-0.
- Friedman JM, Arbiser J, Epstein JA, et al. Cardiovascular disease in neurofibromatosis 1: report of the NF1 Cardiovascular Task Force. Genet Med Off J Am Coll Med Genet. 2002;4(3):105-111. doi:10.1097/00125817-200205000-00002.
- Neiman HL, Mena E, Holt JF, Stern AM, Perry BL. Neurofibromatosis and congenital heart disease. Am J Roentgenol Radium Ther Nucl Med. 1974;122(1):146-149. doi:10.2214/ajr.122.1.146.
- Holt JF. 1977 Edward B. D. Neuhauser lecture: neurofibromatosis in children. AJR Am J Roentgenol. 1978;130(4):615-639. doi:10.2214/ajr.130.4.615.
- Carey JC, Laub JM, Hall BD. Penetrance and variability in neurofibromatosis: a genetic study of 60 families. Birth Defects Orig Artic Ser. 1979;15(5B):271-281.
- Schorry EK, Stowens DW, Crawford AH, Stowens PA, Schwartz WR, Dignan PS. Summary of patient data from a multidisciplinary neurofibromatosis clinic. Neurofibromatosis. 1989;2(2):129-134.
- Colley A, Donnai D, Evans DG. Neurofibromatosis/Noonan phenotype: a variable feature of type 1 neurofibromatosis. Clin Genet. 1996;49(2):59-64. doi:10.1111/j.1399-0004.1996.tb04328.x.
- Tonsgard JH, Yelavarthi KK, Cushner S, Short MP, Lindgren V. Do NF1 gene deletions result in a characteristic phenotype? Am J Med Genet. 1997;73(1):80-86. doi:10.1002/(sici)1096-8628(19971128)73:1<80::aid-ajmg16>3.0.co;2-n.
- Ademiluyi SA, Sowemimo GO, Oyeneyin JO. Surgical experience in the management of multiple neurofibromatosis in Nigerians. West Afr J Med. 1989;8(1):59-65.
- Rasko JE, North KN, Favaloro EJ, Grispo L, Berndt MC. Attenuated platelet sensitivity to collagen in patients with neurofibromatosis type 1. Br J Haematol. 1995;89(3):582-588. doi:10.1111/j.1365-2141.1995.tb08367.x.
- Korf BR, Carrazana E, Holmes GL. Patterns of seizures observed in association with neurofibromatosis 1. Epilepsia. 1993;34(4):616-620. doi:10.1111/j.1528-1157.1993.tb00437.x.
- Kulkantrakorn K, Geller TJ. Seizures in neurofibromatosis 1. Pediatr Neurol. 1998;19(5):347-350. doi:10.1016/s0887-8994(98)00075-7.
- Holmes MD. The causes of epilepsy: common and uncommon causes in adults and children. Arch Neurol. 2012;69(9):1212-1213. doi:10.1001/archneurol.2012.1266.
- North KN, Riccardi V, Samango-Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48(4):1121-1127. doi:10.1212/wnl.48.4.1121.
- Descheemaeker M-J, Plasschaert E, Frijns J-P, Legius E. Neuropsychological profile in adults with neurofibromatosis type 1 compared to a control group. J Intellect Disabil Res JIDR. 2013;57(9):874-886. doi:10.1111/j.1365-2788.2012.01648.x.
- Ferner RE, Hughes RA, Weinman J. Intellectual impairment in neurofibromatosis 1. J Neurol Sci. 1996;138(1-2):125-133. doi:10.1016/0022-510x(96)00022-6.
- Acosta MT, Gioia GA, Silva AJ. Neurofibromatosis type 1: new insights into neurocognitive issues. Curr Neurol Neurosci Rep. 2006;6(2):136-143. doi:10.1007/s11910-996-0036-5.
- Hyman SL, Arthur Shores E, North KN. Learning disabilities in children with neurofibromatosis type 1: subtypes, cognitive profile, and attention-deficit-hyperactivity disorder. Dev Med Child Neurol. 2006;48(12):973-977. doi:10.1017/S0012162206002131.
- Johnson H, Wiggs L, Stores G, Huson SM. Psychological disturbance and sleep disorders in children with neurofibromatosis type 1. Dev Med Child Neurol. 2005;47(4):237-242. doi:10.1017/s0012162205000460.
- Seminog OO, Goldacre MJ. Risk of benign tumours of nervous system, and of malignant neoplasms, in people with neurofibromatosis: population-based record-linkage study. Br J Cancer. 2013;108(1):193-198. doi:10.1038/bjc.2012.535.
- Madanikia SA, Bergner A, Ye X, Blakeley JO. Increased risk of breast cancer in women with NF1. Am J Med Genet A. 2012;158A(12):3056-3060. doi:10.1002/ajmg.a.35550.
- Walker L, Thompson D, Easton D, et al. A prospective study of neurofibromatosis type 1 cancer incidence in the UK. Br J Cancer. 2006;95(2):233-238. doi:10.1038/sj.bjc.6603227.
- Wang X, Levin AM, Smolinski SE, Vigneau FD, Levin NK, Tainsky MA. Breast cancer and other neoplasms in women with neurofibromatosis type 1: a retrospective review of cases in the Detroit metropolitan area. Am J Med Genet A. 2012;158A(12):3061-3064. doi:10.1002/ajmg.a.35560.
- Sharif S, Moran A, Huson SM, et al. Women with neurofibromatosis 1 are at a moderately increased risk of developing breast cancer and should be considered for early screening. J Med Genet. 2007;44(8):481-484. doi:10.1136/jmg.2007.049346.
- Uusitalo E, Kallionpää RA, Kurki S, et al. Breast cancer in neurofibromatosis type 1: overrepresentation of unfavourable prognostic factors. Br J Cancer. 2017;116(2):211-217. doi:10.1038/bjc.2016.403.
- Evans DGR, O’Hara C, Wilding A, et al. Mortality in neurofibromatosis 1: in North West England: an assessment of actuarial survival in a region of the UK since 1989. Eur J Hum Genet EJHG. 2011;19(11):1187-1191. doi:10.1038/ejhg.2011.113.
- Daly MB, Pilarski R, Berry M, et al. NCCN Guidelines Insights: Genetic/Familial High-Risk Assessment: Breast and Ovarian, Version 2.2017. J Natl Compr Cancer Netw JNCCN. 2017;15(1):9-20. doi:10.6004/jnccn.2017.0003.
- Guillamo J-S, Créange A, Kalifa C, et al. Prognostic factors of CNS tumours in Neurofibromatosis 1 (NF1): a retrospective study of 104 patients. Brain J Neurol. 2003;126(Pt 1):152-160. doi:10.1093/brain/awg016.
- Salvi PF, Lorenzon L, Caterino S, Antolino L, Antonelli MS, Balducci G. Gastrointestinal stromal tumors associated with neurofibromatosis 1: a single centre experience and systematic review of the literature including 252 cases. Int J Surg Oncol. 2013;2013:398570. doi:10.1155/2013/398570.
- Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30(1):90-96. doi:10.1097/01.pas.0000176433.81079.bd.
- Doser K, Andersen EW, Kenborg L, et al. Clinical characteristics and quality of life, depression, and anxiety in adults with neurofibromatosis type 1: A nationwide study. Am J Med Genet A. 2020;182(7):1704-1715. doi:10.1002/ajmg.a.61627.
- Pasini A, Lo-Castro A, Di Carlo L, et al. Detecting anxiety symptoms in children and youths with neurofibromatosis type I. Am J Med Genet Part B Neuropsychiatr Genet Off Publ Int Soc Psychiatr Genet. 2012;159B(7):869-873. doi:10.1002/ajmg.b.32095.
- Barton B, North K. The self-concept of children and adolescents with neurofibromatosis type 1. Child Care Health Dev. 2007;33(4):401-408. doi:10.1111/j.1365-2214.2006.00717.x.
- Ablon J. Gender response to neurofibromatosis 1. Soc Sci Med 1982. 1996;42(1):99-109. doi:10.1016/0277-9536(95)00076-3.
- Leschziner GD, Golding JF, Ferner RE. Sleep disturbance as part of the neurofibromatosis type 1 phenotype in adults. Am J Med Genet A. 2013;161A(6):1319-1322. doi:10.1002/ajmg.a.35915.
- Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6):540-545. doi:10.1093/sleep/14.6.540.
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193-213. doi:10.1016/0165-1781(89)90047-4.
- Vranceanu A-M, Merker VL, Park E, Plotkin SR. Quality of life among adult patients with neurofibromatosis 1, neurofibromatosis 2 and schwannomatosis: a systematic review of the literature. J Neurooncol. 2013;114(3):257-262. doi:10.1007/s11060-013-1195-2.
- Vranceanu A-M, Merker VL, Park ER, Plotkin SR. Quality of life among children and adolescents with neurofibromatosis 1: a systematic review of the literature. J Neurooncol. 2015;122(2):219-228. doi:10.1007/s11060-015-1725-1.
- Garwood MM, Bernacki JM, Fine KM, Hainsworth KR, Davies WH, Klein-Tasman BP. Physical, cognitive, and psychosocial predictors of functional disability and health-related quality of life in adolescents with neurofibromatosis-1. Pain Res Treat. 2012;2012:975364. doi:10.1155/2012/975364.
- Fertitta L, Bergqvist C, Armand ML, et al. Quality of life in neurofibromatosis 1: development and validation of a tool dedicated to cutaneous neurofibromas in adults. J EurAcadDermatolVenereol 2022. doi:10.1111/jdv.18140.
- Rasmussen SA, Yang Q, Friedman JM. Mortality in neurofibromatosis 1: an analysis using U.S. death certificates. Am J Hum Genet. 2001;68(5):1110-1118. doi:10.1086/320121.
- Zöller M, Rembeck B, Akesson HO, Angervall L. Life expectancy, mortality and prognostic factors in neurofibromatosis type 1. A twelve-year follow-up of an epidemiological study in Göteborg, Sweden. Acta Derm Venereol. 1995;75(2):136-140. doi:10.2340/0001555575136140.
- Duong TA, Sbidian E, Valeyrie-Allanore L, et al. Mortality associated with neurofibromatosis 1: a cohort study of 1895 patients in 1980-2006 in France. Orphanet J Rare Dis. 2011;6:18. doi:10.1186/1750-1172-6-18.
- Dombi E, Baldwin A, Marcus LJ, et al. Activity of Selumetinib in Neurofibromatosis Type 1–Related Plexiform Neurofibromas. https://doi.org/10.1056/NEJMoa1605943. doi:10.1056/NEJMoa1605943.
- Weiss BD, Wolters PL, Plotkin SR, et al. NF106: A Neurofibromatosis Clinical Trials Consortium Phase II Trial of the MEK Inhibitor. Mirdametinib (PD-0325901) in Adolescents and Adults With NF1-Related Plexiform Neurofibromas. J Clin Oncol Off J Am Soc Clin Oncol. 2021;39(7):797-806. doi:10.1200/JCO.20.02220.
- Gross AM, Wolters PL, Dombi E, et al. Selumetinib in Children with Inoperable Plexiform Neurofibromas. N Engl J Med. 2020;382(15):1430-1442. doi:10.1056/NEJMoa1912735.
- Fisher MJ, Shih C-S, Rhodes SD, et al. Cabozantinib for neurofibromatosis type 1-related plexiform neurofibromas: a phase 2 trial. Nat Med. 2021;27(1):165-173. doi:10.1038/s41591-020-01193-6.